One of the significant differences between a Professional Steel pan and most other pans sold in the market place today is the rate of heat transfer from the stove top to the surface of the pan. The metal substrate for all Professional Steel pans is heavy gauge carbon steel, which heats up very quickly due to its particular thermal properties. Other more commonly used metals for cookware, such as stainless steel and aluminum, do not heat up as quickly as carbon steel. This is primarily because stainless steel conducts heat very unevenly with a number of hot spots, while aluminum is not a very dense metal, with a lower heat capacity compared to carbon steel, which means heat is not retained as well and is lost from a pan more quickly.
 One way to demonstrate that a Professional Steel pan has a fast heating effect is to see how quickly a droplet of water on it reaches the stage in boiling called film boiling, otherwise known as the Leidenfrost Effect. It is generally accepted among experienced chefs that the optimum moment to start cooking is once this droplet of water is at the film boiling stage. When a pan is at this temperature a mercury-like ball will form almost immediately, which will hover over the surface rather than making physical contact with it. At this point the pan is ready for oil and sticking will not occur because, just like the mercury ball, food which is added to the pan will glide along the surface of the oil.
One way to demonstrate that a Professional Steel pan has a fast heating effect is to see how quickly a droplet of water on it reaches the stage in boiling called film boiling, otherwise known as the Leidenfrost Effect. It is generally accepted among experienced chefs that the optimum moment to start cooking is once this droplet of water is at the film boiling stage. When a pan is at this temperature a mercury-like ball will form almost immediately, which will hover over the surface rather than making physical contact with it. At this point the pan is ready for oil and sticking will not occur because, just like the mercury ball, food which is added to the pan will glide along the surface of the oil.
There are a number of benefits for a pan which can reach the stage of film boiling quickly. First, it saves time and energy, and secondly, it is advantageous for human health because the faster food is cooked, the more vitamins are retained within it. However, the major benefit of a fast heating effect is that a pan can produce the high heat needed to develop flavour on food more quickly. The types of cooking methods that require a high heat include searing, sautéing, grilling and stir frying.
The temperature at which film boiling begins to occur is not generally agreed upon, but a rough estimate is 193C. This is just above the ideal temperature needed for browning and caramelizing on meat and vegetables. Other cookware materials such as stainless steel and aluminum can still reach the film boiling stage to sear food, such as chicken breasts, a brown crispy crust. However, compared to a Professional Steel pan these types of materials for cookware will take longer to do so, and the extra cooking time will result in greater moisture loss for a less juicy, drier piece of chicken.
The description film boiling is actually a bit of a misnomer because by the time the droplet of water has reached this stage on the pan there is no longer any actual boiling; there is instead a layer of vapor between the droplet of water and the pan surface. In order to understand why this happens it is first necessary to explain the process of boiling water. As Jearl Walker of Cleveland State University explains, who has studied and written about the process and effects of the Leidenfrost effect in much detail, the initial stages of water boiling actually begin at the bottom of the pan:
“As the water warms, air molecules are driven out of solution in the water, collecting as tiny bubbles in crevices along the bottom of the pan. The air bubbles gradually inflate, and then they begin to pinch off from the crevices and rise to the top surface of the water. As they leave, more air bubbles form in the crevices and pinch off, until the supply of air in the water is depleted.”
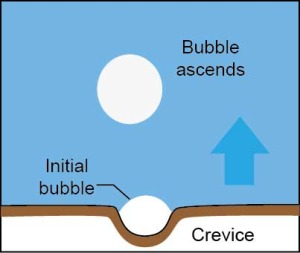 So the formation of air bubbles that can be seen at the beginning of boiling water actually have nothing to do with boiling, they are just a sign that the water is heating. As the pan’s temperature increases the bottom layer of water begins to vaporize, with water molecules gathering in small vapor bubbles in the now dry crevices, which then gradually expand upwards towards the surface of the water. However the boiling stage has still not yet begun as the expanding bubbles collapse before they can reach the surface because the water is not hot enough. Eventually as the pan’s temperature increases further and the water does become hot enough for the expanding bubbles to reach the surface, the boiling process begins when the bubbles pop open with a large splash.
So the formation of air bubbles that can be seen at the beginning of boiling water actually have nothing to do with boiling, they are just a sign that the water is heating. As the pan’s temperature increases the bottom layer of water begins to vaporize, with water molecules gathering in small vapor bubbles in the now dry crevices, which then gradually expand upwards towards the surface of the water. However the boiling stage has still not yet begun as the expanding bubbles collapse before they can reach the surface because the water is not hot enough. Eventually as the pan’s temperature increases further and the water does become hot enough for the expanding bubbles to reach the surface, the boiling process begins when the bubbles pop open with a large splash.
As the boiling process intensifies and the water temperature increases further the vapor bubbles become so abundant that they coalesce into columns of vapor that violently churn upwards and interact with previously detached ‘slugs’ of vapor. This stage of the boiling process is called Nucleate Boiling. It is during this stage that any increase in pan temperature will also increase the rate of heat transfer between the pan and the water.
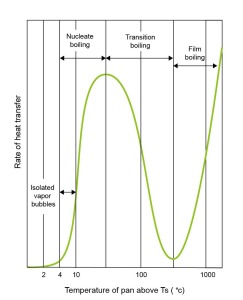 So far, the above is an explanation of the process of boiling water in a large volume, such as in a saucepan full of water. However, if the temperature of the pan goes above 100 degrees, and instead of a large volume of water, it is just in the form of small droplets then these will start to hiss when touching the pan and evaporate quickly. At this point the boiling enters a new phase called Transition boiling. Although during this phase the water evaporates faster as the temperature of the pan increases, the rate of heat transfer actually decreases. This is because some of the bottom surface of the water is now covered by a layer of vapour. Vapour conducts heat much more slowly than water.
So far, the above is an explanation of the process of boiling water in a large volume, such as in a saucepan full of water. However, if the temperature of the pan goes above 100 degrees, and instead of a large volume of water, it is just in the form of small droplets then these will start to hiss when touching the pan and evaporate quickly. At this point the boiling enters a new phase called Transition boiling. Although during this phase the water evaporates faster as the temperature of the pan increases, the rate of heat transfer actually decreases. This is because some of the bottom surface of the water is now covered by a layer of vapour. Vapour conducts heat much more slowly than water.
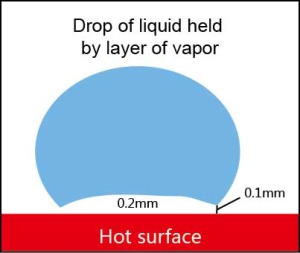 Eventually, as the pan is heated up even further the whole of the bottom surface of the water droplet is covered with vapor. Then energy is transferred to the liquid above the vapor by radiation and gradual conduction. This phase is now film boiling, which as was explained previously is when a pan is at the ideal temperature for cooking.
Eventually, as the pan is heated up even further the whole of the bottom surface of the water droplet is covered with vapor. Then energy is transferred to the liquid above the vapor by radiation and gradual conduction. This phase is now film boiling, which as was explained previously is when a pan is at the ideal temperature for cooking.
A Professional Steel pan can reach the film boiling stage very quickly because of the thermal properties of carbon steel. Moreover, because of the high density of the 2.0mm heavy gauge steel used in Professional Steel pans, when food is added, the temperature will not drop significantly and the searing process of browning and caramelizing can be maintained. This is especially useful because other materials for cookware, such as aluminum or stainless steel, which have either a lower density or poorer heat conduction than carbon steel will require a constant re-adjustment of heat to get the pan up to a higher temperature. If this happens the cooking process will be prolonged, for drier, and less delicious food.


