Pan frying a Premium Cut of Steak
To prepare and cook the perfect steak is not easy. Getting it to the perfect doneness consistently can be a challenge – even for a professional. There are many factors to consider when preparing to cook a delicious, tender steak.
These include:
- the extent of marbling in the steak,
- how it was aged and for how long,
- from which part of the cow was the steak cut,
- the firmness of the steak when touched
- tempering of the steak and:
- pat drying, seasoning and oiling the steak prior to cooking.
How a steak is cooked in the pan and how it is handled afterwards can also determine how delicious the steak is to eat, such as:
- How often the steak is flipped and
- The resting period of the steak after cooking
So, to pan-fry a steak to taste tender and succulent it needs a lot of care and attention. If consumers, in their eagerness to enjoy a good steak, just unwrapped the steaks straight from the refrigerator and immediately dropped them into a pan the steak would not taste nearly as good than if it were properly taken care of before, during and after the cooking process.
However, in addition to the quality of the steak and the care and attention given to it, another important factor is the material of the frying pan. If the pan used to sear the steak does not have the correct thermal properties for heating, then even a well-aged, rib-eye steak with excellent marbling flipped in the correct way, and with a sufficient resting period, may not taste as succulent and flavourful as if another type of pan had been used.
One of the most popular metals for cookware utensils, such as a frying pan, around the world, is aluminum. Aluminum has a number of cooking advantages which includes: lightweight, even heating, and fairly good corrosion resistance. However, for cooking a premium cut of steak, how does the heating effect of an aluminum pan compare to that of carbon steel?
When adding a steak to a frying pan it is important that it begins searing at a high heat almost immediately. In order for a high heat to be maintained after the steak has been added the metal substrate of the pan needs to have a thermal capacity large enough to minimise loss of heat. If the temperature of the pan decreases significantly then the steak will not sear and develop a deep brown crust on the surface. Instead, the steak will steam in its own juices and will be drier and less juicy because of the extra time needed to cook the steak to the correct doneness. This would then negate all the benefits that come with the various types of steak preparation mentioned above, such as the extra expense for a lot of marbling in the steak, or choosing a steak which has been well-aged or hung prior to packing.
So, how does a pan retain most of its heat after a thick premium cut of steak has been added to start the process of searing immediately? There are two important factors in determining this: thickness and the specific heat capacity of the pan. We will come to the importance of thickness later. First we will concentrate on the specific heat capacities of aluminum and carbon steel.
Various materials differ in their ability to store heat; carbon steel and aluminum differ quite markedly. The scientific term used to quantify a material’s heat storage capabilities is called Specific Heat. This is the amount of heat it takes to raise one unit of a substance by one degree. If you look at the left side of the table below you will see that aluminum has a better ability at storing heat than carbon steel.
In fact, the specific heat comparison is a little misleading because it does not take into account the density of each metal. As you can see in the middle part of the table, carbon steel is roughly three times denser than aluminum. As the right-hand side table quite clearly shows, this difference in density then has a big impact on the specific heat per cubic centimeter of both metals, with carbon steel now having a significantly higher specific heat than aluminum. Therefore, assuming the thickness of both metals is the same, the heat capacity (the total heat holding capability of an entire piece of cookware) of a carbon steel frying pan is much greater than that of an aluminum pan.
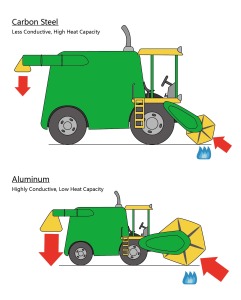 One way to illustrate this difference in heat capacity between a carbon steel and an aluminum pan would be to imagine them as combine harvesters. As the picture below demonstrates, the combine harvester, representing the thermal properties of an aluminum pan, can conduct a large amount of heat very quickly. However, because the combine harvester for aluminum is not very dense (so less material for a smaller combine harvester) it cannot hold as much heat as the far denser carbon steel combine harvester (more material for a bigger combine harvester).
One way to illustrate this difference in heat capacity between a carbon steel and an aluminum pan would be to imagine them as combine harvesters. As the picture below demonstrates, the combine harvester, representing the thermal properties of an aluminum pan, can conduct a large amount of heat very quickly. However, because the combine harvester for aluminum is not very dense (so less material for a smaller combine harvester) it cannot hold as much heat as the far denser carbon steel combine harvester (more material for a bigger combine harvester).
The purpose of these illustrations is to demonstrate that when a steak is added to a high heat capacity carbon steel pan the temperature of it will not drop significantly. Because of the extra mass of the carbon steel pan it can stay hotter for longer than the lower heat capacity aluminum pan which loses heat to its surroundings far more easily. The extra heat stored in the carbon steel pan ensures faster browning and therefore higher moisture retention in the steak after it has finished cooking.
However, in addition to the heat capacity of a pan, the other important factor, which mentioned above and which affects whether a pan can maintain a high temperature for searing a steak quickly to its proper doneness, is its thickness. A carbon steel pan may have a higher specific heat per cubic centimeter than an aluminum pan, but if the gauge of the steel is too thin then the pan will have hot spots and then the steak will be cooked unevenly. A carbon steel pan that is produced is at a thin gauge will conduct heat significantly more in the centre of the pan than at its outer edges. In order to disperse the heat more evenly around the pan all Professional Steel pans are made with 2.0mm gauge. As is shown in the picture below, a thicker gauge broadens the area at which the heat reaches the surface of the pan.
So heavy gauge Professional Steel pans not only have excellent heat retention capabilities but they can also disperse heat evenly around the pan so that a steak can be cooked properly to the correct doneness.
 Currently, heavy gauge Professional Steel frying pans, with the Fiona silicone handle, are sold in many countries throughout the world. All Professional Steel pans are produced at a gauge of 2.0mm. When you consider the density of carbon steel this is very thick and when compared to most aluminum pans quite heavy (making them more durable and less likely to warp). A popular size for this pan is 26cm. By knowing the diameter of the pan, along with the information listed in the specific heat table above, we can calculate the difference in heat capacity between a Professional Steel Fiona frying pan and an aluminum pan with the same measurements (26cm diameter/ 2mm thickness).
Currently, heavy gauge Professional Steel frying pans, with the Fiona silicone handle, are sold in many countries throughout the world. All Professional Steel pans are produced at a gauge of 2.0mm. When you consider the density of carbon steel this is very thick and when compared to most aluminum pans quite heavy (making them more durable and less likely to warp). A popular size for this pan is 26cm. By knowing the diameter of the pan, along with the information listed in the specific heat table above, we can calculate the difference in heat capacity between a Professional Steel Fiona frying pan and an aluminum pan with the same measurements (26cm diameter/ 2mm thickness).
Heat Capacity
Professional Steel Pan = 127.8 J/cm^3 K
Aluminum Pan = 81.8 J/cm^3 K
For the Professional Steel Fiona fry pan the heat capacity is 127.8 J/cm^3 K. In contrast, for an aluminum pan at the same diameter and height the heat capacity would be 81.8 J/cm^3 K. So, as these figures demonstrate, a carbon steel pan stores heat much better than an aluminum pan. A pan with a high heat capacity is very useful for searing meat, such as a steak, when the amount of time spent in the pan will affect how moist and tender it will be after cooking. If a steak is cooked with the heat capacity of an aluminum pan then the extra cooking time needed to get the steak to the proper doneness will result in it not being as moist or tender as it could have been had a heavy gauge Professional Steel pan been used.