In an earlier post shown here, it was demonstrated that Professional Steel pans are more durable than aluminum and cast iron for use and storage in a kitchen (though there is little difference in durability between a Professional Steel pan and a stainless steel pan). Another important aspect of a pan’s durability is whether it can withstand plastic deformation (warping) from the heat source during continual use. How does a Professional Steel pan compare to other metal substrates for this type of durability?
The problems of warping
A quick search on the internet will reveal that the number one complaint about carbon steel pans is their tendency to warp easily. Consumers often complain that the central area of their carbon steel pan has expanded upwards, so that oil and other liquids slide to the side of the pan. Conversely, another problem mentioned is that after some use the centre has bulged downwards, so that the pan no longer lies stable on a flat cooking surface. This is caused by the heat source expanding the edges of the pan base upwards. So warping can make cooking on a pan either difficult, because the ingredients collect around the outer edge, or dangerous, because the pan is not stable on the stove top. But if, as explained in the earlier post, carbon steel pans are so much stronger and tougher than aluminum pans, why do they deform so easily from heating on a stove top? And what can be done to stop it happening?
Why do carbon steel pans warp?
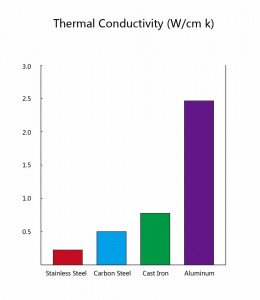 Throughout the world today aluminum is the most popular metal substrate for pans used for dry-heat cooking, such as frying pans. There are two main reasons for this, the first of which is shown in the chart on the right. Of the four most popular materials for cookware – aluminum, cast iron, carbon steel and stainless steel – it is aluminum which has by far the best thermal conductivity. This is why aluminum is such a popular material for frying pans: because it heats up so evenly, it has few hot spots.
Throughout the world today aluminum is the most popular metal substrate for pans used for dry-heat cooking, such as frying pans. There are two main reasons for this, the first of which is shown in the chart on the right. Of the four most popular materials for cookware – aluminum, cast iron, carbon steel and stainless steel – it is aluminum which has by far the best thermal conductivity. This is why aluminum is such a popular material for frying pans: because it heats up so evenly, it has few hot spots.
 The second main reason for the popularity of aluminum as a material for dry-heat cooking pans is that its even heating and less reactive surface make it one of the most suitable materials for coating with the non-stick polymer PTFE (Polytetrafluoroethylene). Ever since this momentous technological discovery was made in 1957, by a man named Marc Gregoire, the majority of pans for dry-heat cooking sold throughout the world have been made of aluminum with a PTFE non-stick coating.
The second main reason for the popularity of aluminum as a material for dry-heat cooking pans is that its even heating and less reactive surface make it one of the most suitable materials for coating with the non-stick polymer PTFE (Polytetrafluoroethylene). Ever since this momentous technological discovery was made in 1957, by a man named Marc Gregoire, the majority of pans for dry-heat cooking sold throughout the world have been made of aluminum with a PTFE non-stick coating.
For general cooking usage, an aluminum pan has to be used over a medium-high heat setting. Although aluminum does conduct heat very evenly around the pan, its low density means that it cannot retain that heat very effectively. If the pan temperature is too low the food will just steam instead of being seared.
A carbon steel pan, on the other hand, is three times more dense and has a much higher heat capacity, and so does not need a medium-high heat setting in order to retain heat. More importantly, these pans actually require a low-medium heat setting, because anything warmer will warp them. As noted above, carbon steel has a much lower heat conductivity than aluminum and on a medium-high setting cannot spread the heat to the sides quickly enough to prevent the centre warping. Most consumers are not aware of this fundamental quality of carbon steel pans and use them on the same heat settings as aluminum pans – which is why so many carbon steel pans warp.
In order to explain why a carbon steel pan needs to be heated at a low-medium heating, and why aluminum can be used on a medium-high setting, we can imagine these pans as a water piping system. The heat is water and the metals are like water pipes of the same diameter. Silver is the most thermally conductive metal. It would be like a completely unclogged water pipe, where a certain amount of water can flow through it per second. As shown previously, aluminum has quite good heat conductivity so it would be like a half-clogged pipe. Carbon steel in contrast because of its lower thermal conductivity would be a more clogged up pipe, with stainless steel as the most clogged.
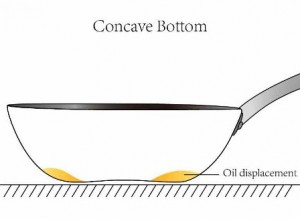
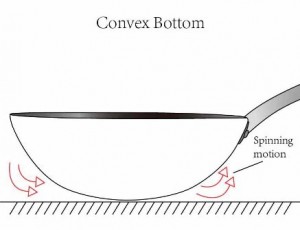
If you overstuff water pipes, the water pressure will build up until the pipe material breaks, and the water pipe bursts. In a carbon steel cookware context, if you heat it faster than the metal can spread it out, you develop hot spots and thus get uneven heating. So if the concavity of the carbon steel pan is set too high then the overheated pan will expand in the centre of the pan. If on the other hand the concavity is too little, the heat will expand towards the edge of the pan and the shape will become convex, with the resulting spinning motion.
Heating method to reduce possibility of a carbon steel pan warping
So for a carbon steel cookware pan with a large diameter but flat base (such as a frying pan or paella pan, rather than a stir wok), one way to deal with the relatively poor thermal conductivity of carbon steel is to pre-heat it at a low-medium heat setting. This gives the heat time to build up and spread out evenly in the pan. Carbon steel is particularly suitable for this slow pre-heating because it is a very dense metal – its poor thermal conductivity is actually an advantage because it can retain heat much better than an aluminum pan. For dry-heat cooking, which requires the pan to maintain a high heat for browning and searing food, a hot pan is essential. When food is added to an aluminium pan, with its low density and limited heat capacity, much of the heat will be absorbed by the food and the temperature of the pan will drop significantly.
A Professional Steel pan is not only three times denser than an aluminum pan, but is generally produced at a heavy gauge of at least 2.0mm. This thickness, along with the highly dense structure of the steel, means the pan can retain a lot of heat, so that its temperature will not drop significantly when food is added – unike an aluminum pan.
Do other cookware materials warp?
As already noted, of the four metals examined here stainless steel is the poorest heat conductor. Because of this all stainless steel pans are fitted with an aluminum disk to disperse the heat to the edges more quickly. Cast iron also has poor thermal conductivity, but because it is so thick it needs to be pre-heated for a much longer time than the other three metals, which means that by the time it is finally ready for cooking most of the heat should have distributed relatively evenly around the pan. For these reasons neither a stainless steel pan nor a cast iron one warps easily, even if heated to a medium-high temperature.
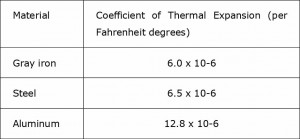
The fact that aluminum can disperse medium-high temperature to the edges of the pan quickly doesn’t mean that it is not affected by warping. In fact, consumers not only complain about aluminum pans denting easily, as noted in the earlier post, but also about warping. The reason for this is not the same as carbon steel, because as we know, even at a medium-high heat setting aluminum heats very evenly with few hot spots. The reason is rather to do with aluminum’s rate of thermal expansion. As shown in the chart below, when heated, aluminum expands three times as much as carbon steel. But whereas carbon steel, due to its high density and heat capacity, can reduce the chance of warping by using a low-medium heat setting, an aluminum pan can only be used at a higher temperature. An aluminum pan at low-medium heat cannot as effectively sear or brown food to impart a delicious caramelized crust – but at the necessary higher temperature it will often warp.
Summary
Professional Steel pans have excellent durability in the kitchen. Not only can they be used and stored with little fear that they might dent, deform or fracture – if heated correctly they can also be used without much fear of warping*. Professional Steel pans have a high heat capacity, so can be pre-heated on a low-medium heat setting to build up and store heat in the pan. Food, such as a steak, can then be added without the temperature of the pan being significantly affected and the browning and searing process can begin immediately. Aluminum pans on the other hand need a medium-high setting because, unlike Professional Steel pans, they lose heat quite easily. If not used on a medium-high heat setting the food will more likely steam rather than sear, and so will take longer to cook, with a drier, less juicy taste as a result. However, this medium-high heat setting will have consequences for the durability of an aluminium pan. Since aluminum has a high rate of thermal expansion, medium-high heat will eventually warp the base of the pan. The result will either be an upward concave bulge with food and liquids sliding to the edges, or a convex downward bulge reducing stability. Either way, once this plastic deformation reaches a certain point the aluminum pan will be obsolete for further usage.
*In order to further enhance a Professional Steel pan’s resistance to warping, the Stable Concave design can be added to the base of the pan. For more information about this highly innovative feature, wholly designed and developed for Professional Steel pans, please read here.